Grand Pharmaceutical Group Limited (stock code: 00512.HK), abbreviated as "Grand Pharma," is an international pharmaceutical and health industry group driven by technological innovation. Its business coverage includes 7 major business fields including cardiovascular emergency, nuclear medicine anti-tumor diagnosis and treatment, cerebro-cardiovascular precision intervention diagnosis, respiratory and critical and severe diseases, eye, nose & throat (ENT), active pharmaceutical ingredient (API) and high-quality amino acids. The group adheres to the strategy of "global development and differentiated innovation" and is committed to providing precise and high-quality medical solutions for patients worldwide.
Grand Pharma has a diverse product portfolio, covering more than 1000 products across various categories, including more than 700 chemical formulations (approved by NMPA), over 130 APIs, over 110 food additives and other chemical products, and over 40 medical devices. More than 130 products are listed in National Essential Medicines List (2018 Edition), and over 260 products are included in the "National Basic Medical Insurance, Work-related Injury Insurance and Maternity Insurance Medicines List" (2024 Edition).
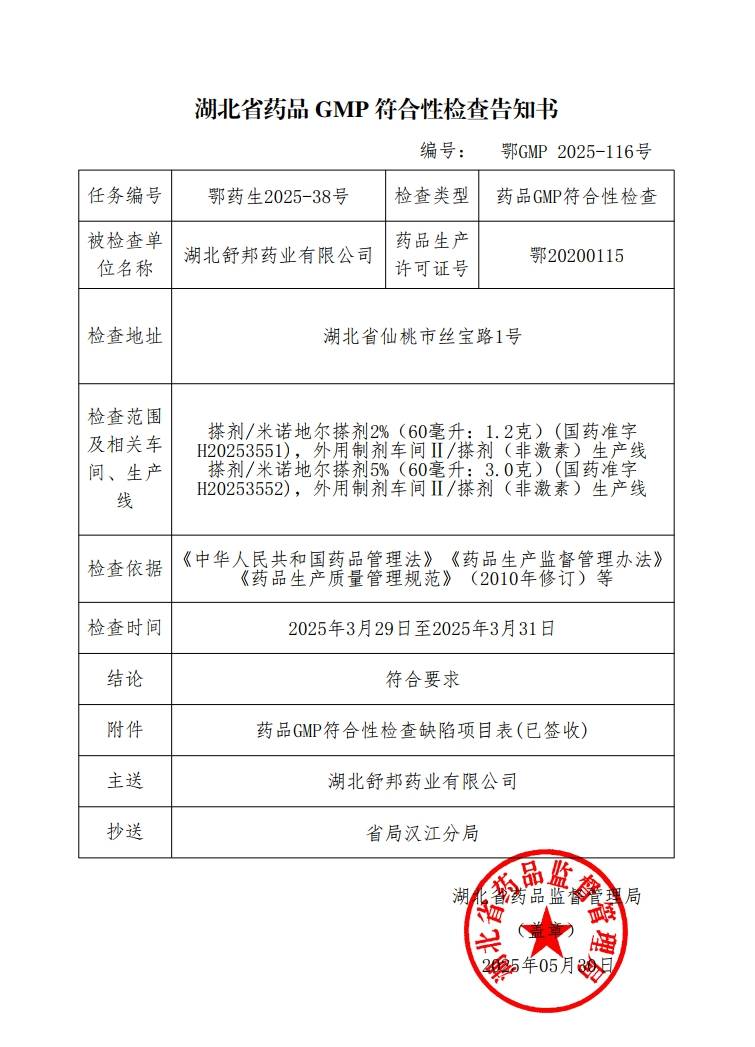
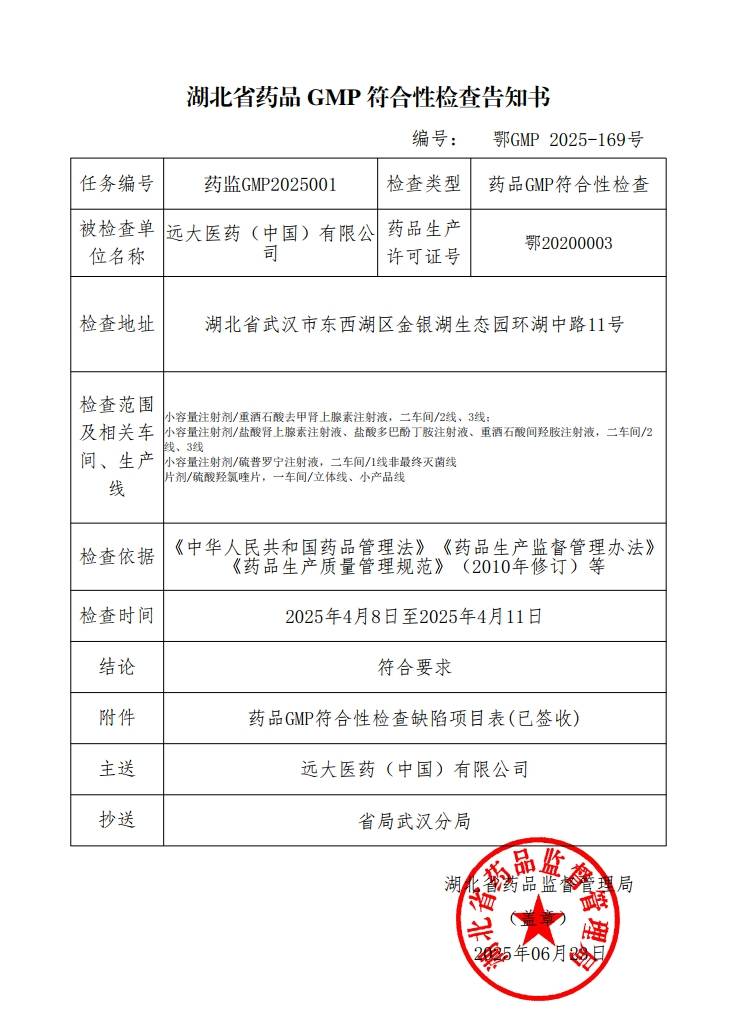
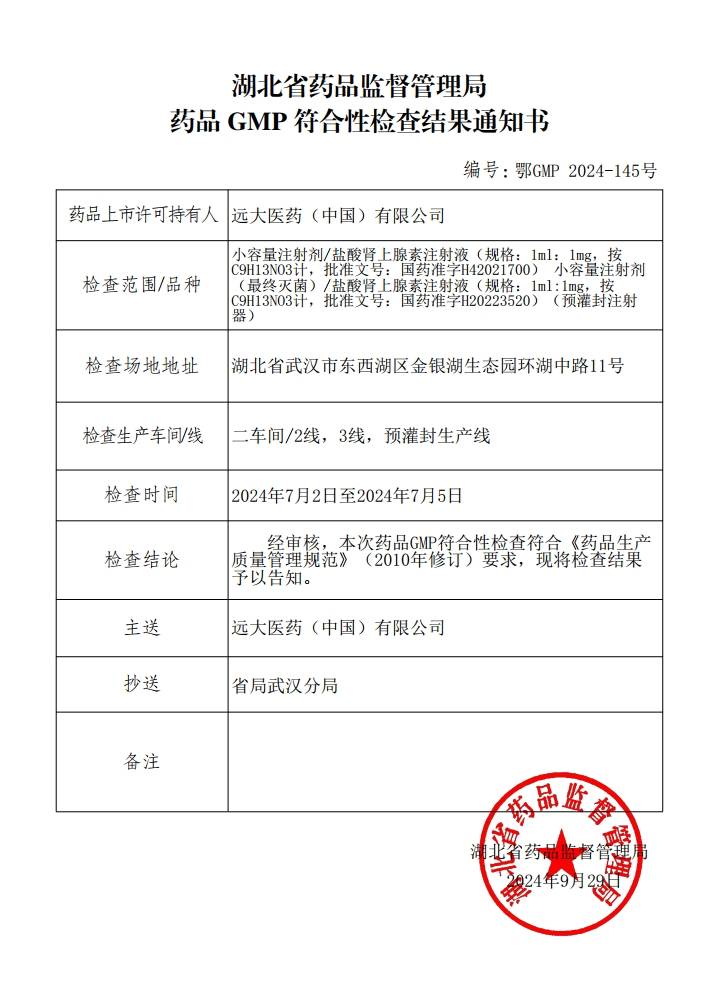
Grand Pharma operates 35 production bases across multiple provinces and cities in China, including Wuhan, Beijing, Tianjin, Shanghai, Chongqing, Chengdu, and Xi'an. It is capable of producing various dosage forms, including but not limited to small- and large volume injections, pre-filled syringes (PFS), lyophilized powder injections, sterile eye drops (BFS, ophthalmic gels), nasal sprays, oral solid dosage forms (tablets, powders, granules, soft/hard capsules), oral solutions, traditional Chinese medicine preparations (capsules, tablets, pills, granules), topical preparations (solutions, creams, ointments, gels, liniments), radiopharmaceuticals, and APIs. The production system has obtained certifications from China GMP, EU EDQM, US FDA, and Japan PMDA. Both finished dosage forms and active pharmaceutical ingredients (APIs) are commercialized in over 30 countries worldwide.
9 R&D centers had been established in Wuhan (focusing on pharmaceuticals and medical devices), Nanjing, Shandong, Chengdu, Shanghai, Changzhou, Australia and the United States, with 147 projects in the pipeline, including 47 innovative projects, focusing on cutting-edge fields such as oncology, gene therapy, and radio pharmaceuticals. Possessing a complete nuclear medicine R&D and production platform and a comprehensive biopharmaceutical technology system.

















 京公网安备 11010802046720号
京公网安备 11010802046720号